www.ncbi.nlm.nih.gov/pmc/articles/PMC6768056/"Journal List PeerJ PMC6768056
As a library, NLM provides access to scientific literature. Inclusion in an NLM database does not imply endorsement of, or agreement with, the contents by NLM or the National Institutes of Health.
Learn more: PMC Disclaimer | PMC Copyright Notice
Logo of peerj
PeerJ. 2019; 7: e7764.
Published online 2019 Sep 27. doi: 10.7717/peerj.7764
PMCID: PMC6768056
PMID: 31579624
~
Dinosaur paleohistology: review, trends and new avenues of investigation
Alida M. Bailleul,corresponding author1,2 Jingmai O’Connor,1,2 and Mary H. Schweitzer3,4,5,6
Academic Editor: Fabien Knoll
Author information Article notes Copyright and License information PMC Disclaimer
Associated Data
Data Availability Statement
Go to:
Abstract
In the mid-19th century, the discovery that bone microstructure in fossils could be preserved with fidelity provided a new avenue for understanding the evolution, function, and physiology of long extinct organisms. This resulted in the establishment of paleohistology as a subdiscipline of vertebrate paleontology, which has contributed greatly to our current understanding of dinosaurs as living organisms. Dinosaurs are part of a larger group of reptiles, the Archosauria, of which there are only two surviving lineages, crocodilians and birds. The goal of this review is to document progress in the field of archosaur paleohistology, focusing in particular on the Dinosauria. We briefly review the “growth age” of dinosaur histology, which has encompassed new and varied directions since its emergence in the 1950s, resulting in a shift in the scientific perception of non-avian dinosaurs from “sluggish” reptiles to fast-growing animals with relatively high metabolic rates. However, fundamental changes in growth occurred within the sister clade Aves, and we discuss this major evolutionary transition as elucidated by histology. We then review recent innovations in the field, demonstrating how paleohistology has changed and expanded to address a diversity of non-growth related questions. For example, dinosaur skull histology has elucidated the formation of curious cranial tissues (e.g., “metaplastic” tissues), and helped to clarify the evolution and function of oral adaptations, such as the dental batteries of duck-billed dinosaurs. Lastly, we discuss the development of novel techniques with which to investigate not only the skeletal tissues of dinosaurs, but also less-studied soft-tissues, through molecular paleontology and paleohistochemistry—recently developed branches of paleohistology—and the future potential of these methods to further explore fossilized tissues. We suggest that the combination of histological and molecular methods holds great potential for examining the preserved tissues of dinosaurs, basal birds, and their extant relatives. This review demonstrates the importance of traditional bone paleohistology, but also highlights the need for innovation and new analytical directions to improve and broaden the utility of paleohistology, in the pursuit of more diverse, highly specific, and sensitive methods with which to further investigate important paleontological questions.
Keywords: Dinosaurs, Birds, Mineralized tissues, Soft-tissues, Molecular paleontology, Paleohistochemistry, Standard paleohistology, New trends..."
~
letterstocreationists.wordpress.com/dinosaur-soft-tissue/"Dinosaur Soft Tissue
Soft Tissue in Dinosaur Fossils: Evidence for a Young Earth?
In an earlier article, “Evidences for a Young Earth”, I discussed a number of proposed physical evidences that the earth is only a few thousand years old, rather than billions of years old. Holding to a literal interpretation of Genesis, young earth creationists circulate about a hundred of these arguments for a young earth. Most of these claims have been around for several decades, and have long since been addressed by mainstream scientists.
A relatively new area of controversy is the discovery of soft tissues in dinosaur fossils. The state of these discoveries changes every few years, so some of the standard science web sites have not kept up. This is a somewhat dramatic topic, which the young earth proponents have appropriated enthusiastically. It is #3 on a list of “10 Best Evidences From Science That Confirm a Young Earth”, according to Answers in Genesis.
A 2009 Institute for Creation Research article by B. Thomas [1] leads off:
In recent decades, soft, squishy tissues have been discovered inside fossilized dinosaur bones. They seem so fresh that it appears as though the bodies were buried only a few thousand years ago.
Since many think of a fossil as having had the original bone material replaced by minerals, the presence of actual bone–let alone pliable blood vessels, red blood cells, and proteins inside the bone–is quite extraordinary. These finds also present a dilemma. Given the fact that organic materials like blood vessels and blood cells rot, and the rates at which certain proteins decay, how could these soft tissues have been preserved for ten thousand, let alone 65 million or more, years?
This sounds amazing – – “fresh”, “soft, squishy tissues” and “pliable blood vessels, red blood cells, and proteins”. This verbiage makes the reader think that someone cracked open these dinosaur bones and found raw tissue flopping around inside, dripping with red blood cells. Is that really the case? Let’s turn to the facts. Here are the topics treated below:
1992: Mary Schweitzer Sees What Looks Like Red Blood Cells
2004: Schweitzer Finds Bits of Soft Tissue in T. Rex Bones
2006 Onward: Sequencing Proteins from Dinosaur Bones
Mechanisms for Ancient Protein Preservation
Osteocyte Cells, Traces of DNA, and Iron as a Preservative
Assessment of Evidence That Soft Tissue Can Persist for 70 Million Years
Invariance of Radioactive Decay Rates
The Dinosaur-Bird Connection
Mary Schweitzer on Creation
Further Findings
Conclusions
APPENDIX: CARBON DATING OF DINOSAUR BONES
************************************************
1992: Mary Schweitzer Sees What Looks Like Red Blood Cells: ..."

"...Key discoveries in this area over the past twenty years have been made by Mary Schweitzer, now a professor of paleontology at North Carolina State University. In 2010, she wrote an article, “Blood From Stone”, in Scientific American which summarized her work to that point [2]. She starts off describing an afternoon in 1992, when she was a graduate student looking in a microscope at a thin bone slice from a newly excavated Tyrannosaurus rex fossil skeleton. She saw what looked like red blood cells – they were “the right size, shape and color to be blood cells, and they were in the right place, too.” This was a surprise, since “The conventional wisdom holds that when an animal dies under conditions suitable for fossilization, inert minerals from the surrounding environment eventually replace all of the organic molecules—such as those that make up cells, tissues, pigments and proteins—leaving behind bones composed entirely of mineral.”
Were these actually red blood cells, or the chemically transformed remains of red blood cells, or merely artifacts of some unknown geological process that produced rounded blobs of material? If these objects were red blood cells, how could organic matter be preserved for more than 65 million years, when the last of the dinosaurs died out? Schweitzer has spent the last two decades trying to answer these questions.
In the mid-1990’s she did a couple of chemical tests with the dinosaur bones. Spectroscopic tests of light wavelengths emitted or absorbed indicated that somewhere in the fossil bones were compounds that were consistent with heme. Heme is a small, relatively stable iron-containing molecule which gives blood its red color and is the key oxygen-carrying component of the hemoglobin protein. Heme has been identified by Greenwalt, et al. in the abdomen of a 46-million-year-old mosquito fossil. Schweitzer noted in a 1997 article [3]:
We also thought hemoglobin could be in the tissue because at its core are structures that have a reputation for durability. Called heme units, these chemically stable structures consist of a ringlike organic compound called porphyrin bound to an iron atom. Porphyrins are an important part of many biological molecules, including chlorophyll, which plants need for photosynthesis. Porphyrins derived from chlorophyll have been found in sediments dating back to the Carboniferous, when vast forests blanketed the planet many millions of years before the dinosaurs existed. So we did not think it too far-fetched that heme units from hemoglobin might still exist in our T. rex.
Also, the responses of immune systems of laboratory mice to injections of powdered dinosaur bones suggested that the bones “contained something similar to the hemoglobin in living animals”[2]. The observed immune response did not require that a full hemoglobin protein was present in the fossil bones, but only a tiny fragment of the protein, “possibly 3-4 amino acids”.
At that point, Schweitzer recounts [2], “We could not show that the hemoglobinlike substance was specific to the red structures—the available techniques were not sufficiently sensitive to permit such differentiation. Thus, we could not claim definitively that they were blood cells. When we published our findings in 1997, we drew our conclusions conservatively, stating that hemoglobin proteins might be preserved and that the most likely source of such proteins was the cells of the dinosaur. The paper got very little notice.”
Just to be clear, her assessment of these objects was that they were not actual red blood cells (e.g. with cell walls or other cellular structures), but rather some chemically transformed remnants of the dinosaur blood [4]:
Clearly these structures are not functional cells. However, one possibility is that they represent diagenetic alteration of original blood remnants, such as complexes of hemoglobin breakdown products, a possibility supported by other data that demonstrate that organic components remain in these dinosaur tissues.
In the next few years, she analyzed some other fossils from the age of dinosaurs, finding evidence for preservation of some keratin proteins from fossil claws and from feather-like fibers. She noted [2] that keratin proteins “are good candidates for preservation because they are abundant in vertebrates, and the composition of this protein family makes them very resistant to degradation.” This work was published in 1999. Again, it was largely ignored, since her findings “challenged everything scientists thought they knew about the breakdown of cells and molecules. Test-tube studies of organic molecules indicated that proteins should not persist more than a million years or so.”
Fuelling the skepticism of her colleagues at that time was the recent debunking of some 1990’s claims of the finding of DNA in dinosaur fossils. In one case, the DNA was found to have resulted from human contamination, and in the other case the DNA came from living fungi and plants, not a reptile [5].
2004: Schweitzer Finds Bits of Soft Tissue in T. Rex Bones
In 2003, Schweitzer received some chunks of T. rex thigh bone (femur), from a recently-excavated fossil skeleton from base of the Hell Creek formation in Montana. This formation has been dated by various radiometric means to about 65-68 million years ago.
In today’s birds, when a female bird is about to lay eggs, it produces distinctive “medullary” bone which serves as a reservoir of calcium for the egg-shells. Schweitzer noticed that the dinosaur thigh bone specimen seemed to include some of this type of bone. For bird bones, one can dissolve the hard mineral part of the bone away in weak acid over a period of weeks, leaving the soft tissue available for examination. In early 2004, Schweitzer asked her technician to treat the T. rex bones in this manner. For the medullary bone they found that this treatment yielded the stretchy, fibrous tissue shown below: ..."

"...When the regular cortical bone was likewise dissolved away with weak acid, a network of what looked like normal, flexible blood vessels was revealed:

"Hollow, branching vessels from demineralized T. rex femur. Source: M. H. Schweitzer, Scientific American, December, 2010, pg. 62. [2]"
"Inside the vessels were suspended what looked like red blood cells:

"...2006 Onward: Sequencing Proteins from Dinosaur Bones
Schweitzer and her colleagues published these photos in the prestigious journal Science in 2005 [6]. She recounts [2]:
The paper garnered a lot of attention, but the scientific community adopted a wait-and-see attitude. We claimed only that the material we found resembled these modern components— not that they were one and the same. After millions of years, buried in sediments and exposed to geochemical conditions that varied over time, what was preserved in these bones might bear little chemical resemblance to what was there when the dinosaur was alive. The real value of these materials could be determined only if their composition could be discerned. Our work had just begun.
Using all the techniques honed while studying [other fossils], I began an in-depth analysis of this T. rex’s bone in collaboration with Asara, who had refined the purification and sequencing methods we used in the mammoth study and was ready to try sequencing the dinosaur’s much older proteins. This was a much harder exercise, because the concentration of organics in the dinosaur was orders of magnitude less than in the much younger mammoth and because the proteins were very degraded. Nevertheless, we were eventually able to sequence them. And, gratifyingly, when our colleague Chris Organ of Harvard compared the T. rex sequences with those of a multitude of other organisms, he found that they grouped most closely with birds, followed by crocodiles— the two groups that are the closest living relatives of dinosaurs.
When the papers detailing this protein sequencing work were published in 2007 [7, 8] and 2008, they generated “a firestorm of controversy”. Because proteins in laboratory experiments degrade relatively quickly, it was believed in the scientific community that original proteins simply could not persist for so many millions of years. Some scientists viciously attacked the protein sequencing techniques of Schweitzer’s collaborator, John Asara of Harvard Medical School [28].
Also, Thomas Kaye, et al. published a paper in 2008 [9] which made a strong case that the flexible stuff found in dinosaur bones was merely a “biofilm” produced by modern bacteria which had invaded the bone pores. Kaye’s team used electron microscopy to examine material from a range of fossils, including some from the same Hell Creek formation which had produced Schweitzer’s T. rex. Doing the same sort of demineralization as Schweitzer, Kaye found similar structures, but interpreted them differently. The photo below shows branching vessels, containing small round red objects, which are about the same size as red blood cells. These objects were identified as “framboids”, which are a small, round deposits of inorganic iron mineral. Framboids are common in various sediments, and typically have nothing to do with red blood cells or other biological origin. Presumably they formed in these pores in the fossil bones by inorganic geochemical processes..."

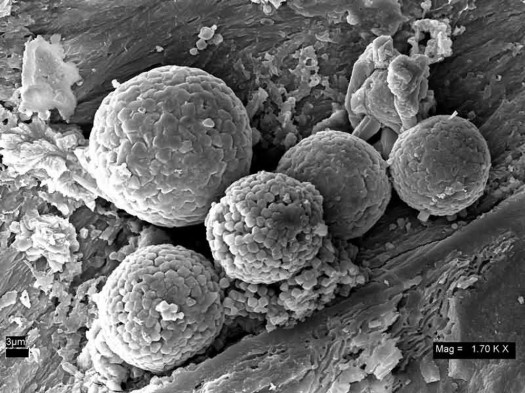
"...Kaye, et al. [9] found that the infrared spectrum from organic material scraped from chambers in a fossil turtle shell (carapace) recovered from the Hell Creek formation better matched a modern bacterial biofilm than collagen protein from a modern chicken tendon. This supported their contention that the soft tissue in the fossils was bacterial in origin, not preserved reptilian tissue. Also, when they submitted some of the soft tissue extracted from fossil bones for carbon-14 dating, the results showed a modern date, again pointing to recent bacterial activity as the origin of these materials.
My opinions, which are subject to correction, on Kaye’s biofilm results [9] are:
( a ) From looking at the electronic micrographs, his identification of the little round red things as spheres of primarily inorganic iron oxide is correct. While they are clearly not red blood cells, the iron may have originated from the dinosaur hemoglobin.
( b) The soft stuff he scraped from the fossil turtle carapace might well be bacterial slime, as he proposed, based on the infrared signature. His paper notes, “A turtle carapace from the Hell Creek formation was selected for spectroscopy because of its proportionally large chambers in the trabecular bone that allowed scraping the coatings loose.” The “large chambers” might have served as accessible habitat for soil bacteria. This environment would differ from the hermetically-sealed, largely mineralized interior pores of dinosaur bones.
( c ) His carbon-14 results, giving post-1950 age to his organic matter, are not consistent with either a 70-million-year-old origin or a 4500-year-old Noahic Flood burial. It is possible that the interior of his particular bone sample was infiltrated by bacteria, or by humic or other inorganic soil acids.
For some months, Kaye’s biofilm thesis seemed more credible to the scientific community than Schweitzer’s contention that the soft tissues were the remnants of actual dinosaur tissue. Schweitzer’s team responded with an in-depth analysis of bones from an 80-million-year-old duckbill hadrosaur, B. canadensis [10]. These fossil bones were exhumed relatively quickly and rushed to the lab for analysis, to minimize exposure to the elements. Her team found the tissues in this second dinosaur were better-preserved than the earlier T. rex. The morphology of these tissues, their immunological behavior, and the sequences of amino acids in fragments of protein were consistent with them being derived from animal (dinosaur) tissue, not from bacteria. Schweitzer writes [2]:
As we had hoped, we found cells embedded in a matrix of white collagen fibers in the animal’s bone. The cells exhibited long, thin, branchlike extensions that are characteristic of osteocytes, which we could trace from the cell body to where they connected to other cells. A few of them even contained what appeared to be internal structures, including possible nuclei.
Furthermore, extracts of the duckbill’s bone reacted with antibodies that target collagen and other proteins that bacteria do not manufacture, refuting the suggestion that our soft-tissue structures were merely biofilms. In addition, the protein sequences we obtained from the bone most closely resembled those of modern birds, just as [the earlier T. rex’s] did. And we sent samples of the duckbill’s bone to several different labs for independent testing, all of which confirmed our results.
The high-resolution protein sequences from this duckbill dinosaur showed it to be more closely related to living birds than to living alligators [10]. This is consistent with evolutionary expectations from the fossil record. Statistical analyses of collagen protein gave a “robust” grouping of the two dinosaurs (the duckbill hadrosaur and the earlier T. rex protein sequences) with birds (ostrich and chicken), but there was not enough sequence data to correctly parse out the relationships among the two dinosaurs and the two birds.
The analyses showed evidence for crosslinking of the proteins, and other chemical modifications (e.g., “unusual complexes between C, N, and Fe”) that are consistent with long-term aging and stabilization of this material. Schweitzer noted [2] that these findings evidently swayed scientific opinion in her direction: “After we reported these findings in Science in 2009, I heard no complaints.”
Nevertheless, some researchers continue to criticize her protein sequencing results, and as of mid-2017 no other research group has been able to detect proteins in dinosaur fossils which can be sequenced to show they are not just contamination from other, modern sources.
Mechanisms for Ancient Protein Preservation
Schweitzer has shifted focus in recent years from simply demonstrating that fragments of protein did survive for millions of years in dinosaur bones, to considering the mechanisms of how this protein was preserved. In a 2005 article [11] she acknowledged that, “Evidence supporting the preservation of endogenous biomolecules in the pre-Cenozoic fossil record has generally been met with skepticism, because it is assumed that primary organic molecules cannot withstand the alterations and breakdown that occur during diagenesis.” However, this skeptical opinion was based on test-tube experiments which may not adequately represent the conditions within the pores of dinosaur bone: “Laboratory experiments designed to approximate molecular diagenesis apply physical and chemical parameters not normally encountered in nature (e.g. pH <1, T >300 ⁰C) and do not account for the protective effects of mineral association. Therefore, their utility as a proxy for diagenetic processes at the molecular level in naturally preserved samples is somewhat limited.” Schweitzer went on to list some 16 earlier studies which showed that “ amino acids, short peptides, and amino sugars can persist within fossils over a wide geological age distribution”, plus 14 studies where “immunological techniques have identified antigenic compounds in fossils of varying ages and from various source taxa.”
Schweitzer published a broad review [12] of “Soft Tissue Preservation in Terrestrial Mesozoic Vertebrates” in 2011. Citing more than 200 studies, she discussed many prior instances of soft tissue finds among Mesozoic fossils. The Mesozoic era is seen as ranging from about 252 to 66 million years ago, and has been termed the “Age of Reptiles”, since they were dominant animals on land, in the air, and in the sea. Most examples of tissue preservation are of the skin and its appendages, including scales, feathers, and claws. These “consist largely of durable and waterproof keratin proteins”. Keratin has high preservation potential “because of its molecular structure, its tendency to form cross-links, and its abundant, nonpolar amino acids.”
However, even these finds are unusual, since the norm is for skin as well as underlying tissue to completely decompose:
Because the carbon and nitrogen that make up proteins, DNA, cells, and tissues of multicellular organisms are useful to microbes for metabolic energy, organic remains are normally degraded rapidly postmortem; indeed, under normal circumstances, more than 99% of the reduced carbon making up these components is returned quickly to the carbon cycle by microbes. Taphonomic experiments show that in most cases where whole carcasses of been deposited on the ground surface, they can be completely skeletonized in as little as 2 to 3 weeks, and degradation-linked changes in cell morphology/chemistry can occur within minutes of death. Consequently, the presence of originally soft tissue components or cells in association with fossilized remains of extinct organisms shows that processes normally involved in degradation have been slowed or arrested soon after death, and before complete decay occurs.
Schweitzer offers some suggestions for how this mitigation of decay may come about. If an animal carcass dries out fairly rapidly, its tissues can undergo changes which render them more stable: “…early desiccation through mummification may make these specimens prime targets for the recovery of biomolecules other than collagen.”
As an aside, many of the eye-catching headlines about “mummified dinosaurs” are misleading. For instance, a 2007 National Geographic article was captioned, ” ‘Dinosaur Mummy’ Found; Has Intact Skin, Tissue “. What you only discover half-way through the article is that the skin and other tissue have been replaced by minerals, so this is not the preservation of organic soft tissue, but rather the preservation of the detailed physical forms of the original soft tissues. This is interesting, but it is also reasonably well-understood, at least in outline. Lingham-Soliara and Glabb [13] reported on their analysis of the microstructure of collagen from decomposed dolphin, python and turtle tissue, which had subsequently been air-dried. They found that, despite this severe exposure to decay and dehydration, “many collagen fibres maintained their structural integrity, showing little degradation”. Also, “re-hydrating the dehydrated tissue showed minimal structural loss”. They concluded that a viable path for the preservation of soft tissue long enough for it to become mineralized (fossilized) would be for an animal to die during a dry spell (which presumably also killed off most scavengers), become mummified, and later get covered with mineral-rich water or sediments.
Schweitzer [12] also mentioned earlier studies which discussed the stabilization of tissues within mineral settings like the pores of bone:
Close association with the mineral phase (Child 1995) may act similarly to chemical fixation (e.g., with formaldehyde), offsetting enzymatic and microbial degradation (Kharalkar et al. 2009 and references therein). This may occur because microbial enzymes are too large for most pores in bone and because the mineral phase of bone forms a barrier to digestion (Trueman & Martill 2002, Turner-Walker 2008). Alternatively it may occur because the small size, large surface area, and reactivity of bone mineral crystals may inhibit enzymatic degradation, in a process similar to that demonstrated for clay grains (Butterfield 1990, 2003). Finally, the constraints of association with mineral may prevent molecular swelling during degradation, ultimately preventing access to more reactive sites on molecules (M. J. Collins, personal communication).
A 2011 study by San Antonio, Schweitzer and others [14] involved the analysis of fragments (peptides) of collagen protein recovered from dinosaur bones, and mapping the results onto molecular models of collagen derived from existing species to determine the configuration of the collagen molecules within the dinosaur bones. The abstract reads, in part:
The dinosaur peptides localized to fibril regions protected by the close packing of collagen molecules, and contained few acidic amino acids. Four peptides mapped to collagen regions crucial for cell-collagen interactions and tissue development. Dinosaur peptides were not represented in more exposed parts of the collagen fibril or regions mediating intermolecular cross-linking. Thus functionally significant regions of collagen fibrils that are physically shielded within the fibril may be preferentially preserved in fossils.
These results show empirically that structure-function relationships at the molecular level could contribute to selective preservation in fossilized vertebrate remains across geological time, suggest a ‘preservation motif’, and bolster current concepts linking collagen structure to biological function. This non-random distribution supports the hypothesis that the peptides are produced by the extinct organisms and suggests a chemical mechanism for survival.
San Antonio, et al. [14] note that extrapolations of glassware studies of protein degradation at accelerated conditions of acidity and high temperature predict that protein strands cannot last more than a few million years (at 10 ⁰C), but suggest that these models may not be appropriate, since “they do not consider the molecules in their native state (i.e., folded, closely-packed, cross-linked or, in the case of bone, stabilized by association with the mineral phase).”
Demarchi, et al. [31] have recently quantified and mechanistically described the stabilization of proteins which are bound to a mineral surface. They found that this surface effect accounts for the survival of original proteins in fossil ostrich egg shells in hot African climates for at least 3.8 million years, which is longer than otherwise expected. They calculated that this corresponds to protein survival for at least 16 million years at a cooler constant temperature of 10 C as would be typical of north-west Europe.
Fazale Rana of Reasons to Believe (an evangelical Christian ministry which accepts an old earth) unpacked the significance of these findings [15] :
Collagen’s basic structural unit is called a triple helix, consisting of three protein chains intertwining around each other. At certain points along the triple helix, the individual protein strands are chemically bound to each other to form crosslinks.
Numerous collagen triple helices assemble in a staggered fashion to form a larger structure called a collagen fibril. Large numbers of collagen fibrils in turn assemble, with the aid of other proteins, into collagen fibers..."










